Lawrence Monoson, CEO - RxData
LUNSUMIO (mosunetuzumab): A Favorable Access Decision in Contrast to the United Kingdom
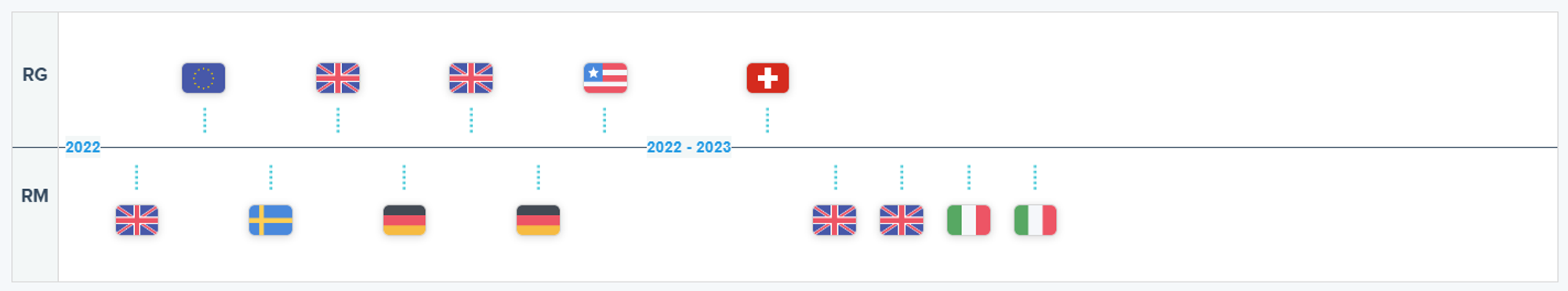
Recent Decisions on LUNSUMIO as Displayed on the RxData Platform
- On September 16, 2023, 470 days post its EC approval, AIFA in Italy assigned LUNSUMIO a Class H status, also stipulating its monitoring within a patient registry. Notably, the FDA approved the drug 202 days after its EMA Marketing Authorization. Therefore, Italian patients gained access to the medication only 268 days post its U.S. approval.
- Germany’s G-BA evaluated the extension 195 days post the EC endorsement, impacting an estimated 650 to 690 patients.
- Whereas accessibility is granted in Italy and Germany, it remains a distant reality for England and Scotland. Post the MHRA's October 2022 approval in the United Kingdom, both England’s NICE and Scotland’s SMC have refrained from recommending its reimbursement, as of May 31, 2023, and September 11, 2023, respectively.
- The CHMP at EMA adopted a positive opinion on April 22, 2022, recommending the granting of a conditional marketing authorization for Roche’s LUNSUMIO for the treatment of relapsed or refractory follicular lymphoma. This decision was sanctioned by the EC on June 3, 2022, with the specified indication being “Lunsumio as monotherapy is indicated for the treatment of adult patients with relapsed or refractory Follicular Lymphoma (FL) who have received at least two prior systemic therapies.”
VOSEVI (sofosbuvir, velpatasvir, and voxilaprevir): Pediatric Hepatitis C Patients Ages 12 to 18 Still Ineligible for Reimbursement in Italy, as Opposed to Other European Countries
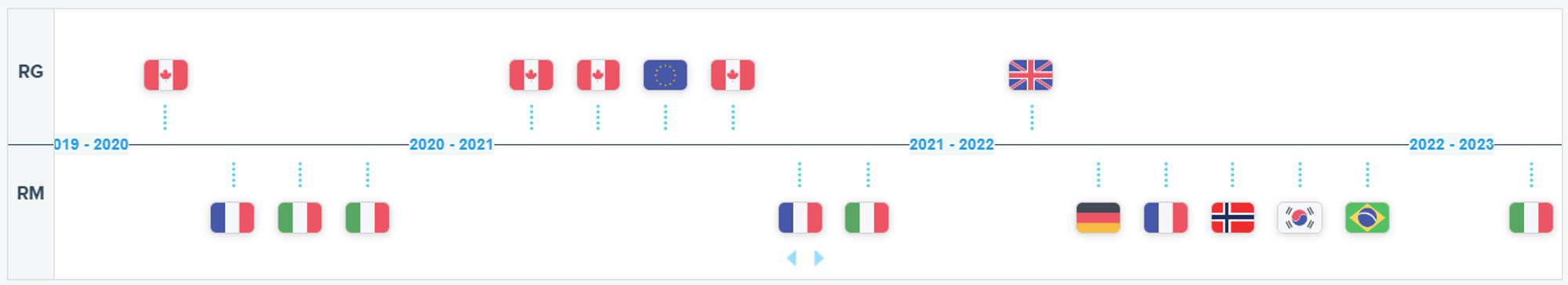
Recent Decisions on VOSEVI as Displayed on the RxData Platform
- On September 17, 2023, a considerable 731 days post its EC approval, AIFA in Italy denied reimbursement for VOSEVI’s new package, categorizing it as Class C, denying pediatric patients access to the drug
- Germany’s G-BA conducted a review of the extension 203 days after EC approval, affecting an estimated 24 to 39 patients.
- France’s HAS also reviewed the extension 216 days post the EC approval and chose to reimburse, with a projected impact on less than 100 patients annually.
- Norway’s Beslutningsforum for nye metoder approved the reimbursement 375 days post the EC approval.
- Contrarily, Pediatric Hepatitis C patients aged 12 to 18 in Italy are left without reimbursement. Patients in Germany, France and Norway are already experiencing the benefits of positive reimbursement resolutions.
- On July 22, 2021, EMA’s CHMP adopted a positive opinion recommending an extension to the indication of Gilead’s VOSEVI (sofosbuvir/velpatasvir/voxilaprevir) to patients aged 12 years and above. The EC adopted the decision on September 16 2021. Previously the indication was approved solely for adults as per the July 26, 2017 Marketing Authorization. The current indication states: “Vosevi is designated for the treatment of chronic Hepatitis C virus (HCV) infection in patients aged 12 years and above, weighing at least 30 kg."
TIBSOVO (ivosidenib): Standard Procedure Yields Class C(nn) Designation, but Unlike in France, Patients in Italy Lack Access through an Early Access Program
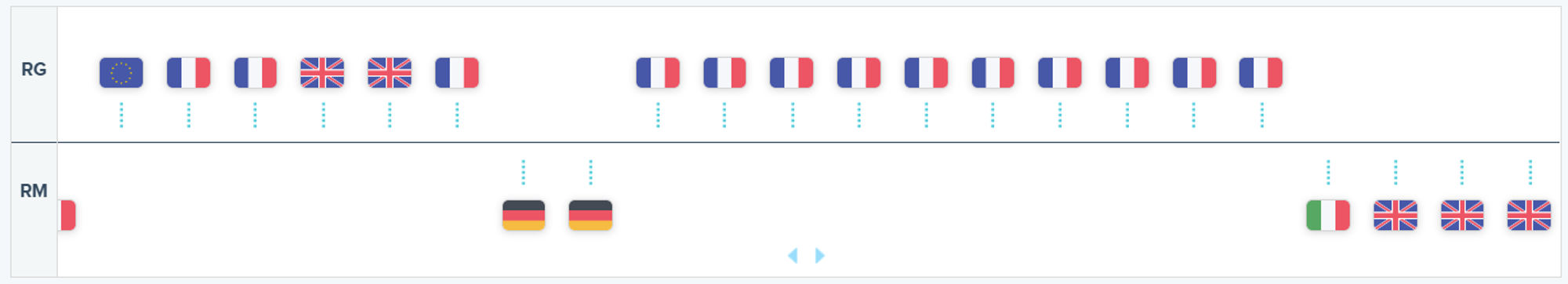
Recent Decisions on TIBSOVO as Displayed on the RxData Platform
- Italy’s AIFA assigned a Class C(nn) to TIBSOVO on September 9, 2023, for the two indications approved by the EC 128 days prior. At the time of writing this article, the drug remains inaccessible via Compassionate Use in Italy, contrasting with France where both indications are available under Early Access Authorization.
Definitions:
- AIFA: Agenzia italiana del farmaco
- Class H: Fully Reimbursed drugs in hospitals
- Class C(nn): EMA authorized drugs, awaiting AIFA pricing and reimbursement assessment, temporarily classified in “class C non-negotiated”, meaning they can be commercialized without being reimbursed by the Italian NHS
- CHMP: Committee for Medicinal Products for Human Use (EMA)
- EC: European Commission
- EMA: European Medicines Agency
- G-BA: German Federal Joint Committee (Gemeinsamer Bundesausschuss)
- MHRA: Medicines and Healthcare products Regulatory Agency
- NICE: National Institute for Health and Care Excellence
- SMC: Scottish Medicines Consortium



